Outstanding Info About How To Check For Sterility

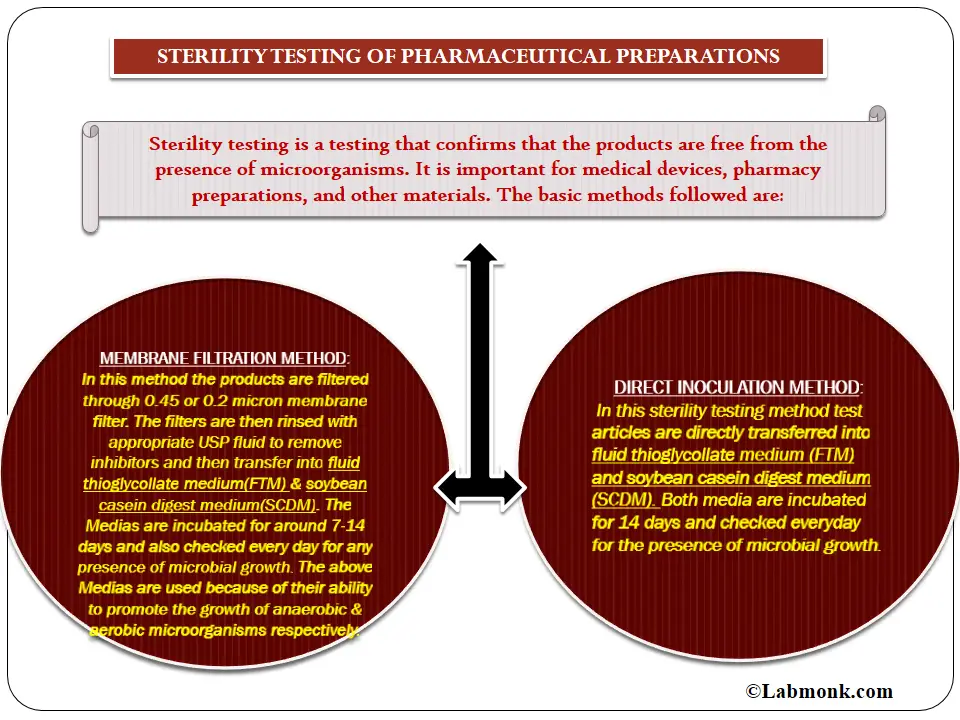
Membrane filtration and direct inoculation.
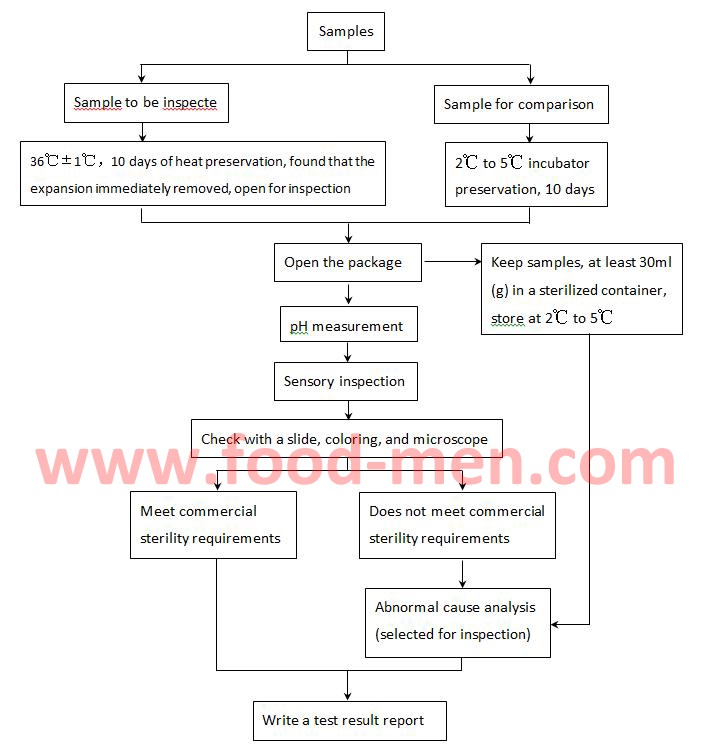
How to check for sterility. Sterility can be defined as the freedom from the presence of viable microorganisms. For presterilized single use funnels a sterility check shall be performed on one funnel per lot. This testing may be performed on 100% of the batch or on representative portions and may be conducted.
Bf testing how to test for b/f: However, the conditions that guarantee absolute sterility are usually too. This testing may be performed on 100% of the batch or on representative portions and may be conducted.
Test methods including those defined in this compendium (sterility tests 〈71〉) utilize a number of samples taken from a large population to infer the “sterility” of the whole. You may have a general physical exam, including a regular gynecological exam. Tests for female infertility try to find out if any of these processes are impaired.
A sterility test attempts to infer the state (sterile or nonsterile) of a batch from the results of an examination of part of a batch, and is thus a statistical operation. There are two recommended methods of sterility testing for pharmaceuticals: The filtration series is considered ended when more than 30 minutes elapses between successive.
Sterilization procedures should be monitored using biological, mechanical, and chemical indicators. The current 21 cfr 610.12(d)(2) states:. It's a fancy way of saying there isn't any cause your doctor can identify for your abnormal or low sperm count.
It is interesting to consider the proposed cber sterility test in regards to sample size. 1.1 causes that trigger sterility: